How Does NormaLyte Work?
Simply put, NormaLyte works in your gut to transport water into your body using a super exact recipe.
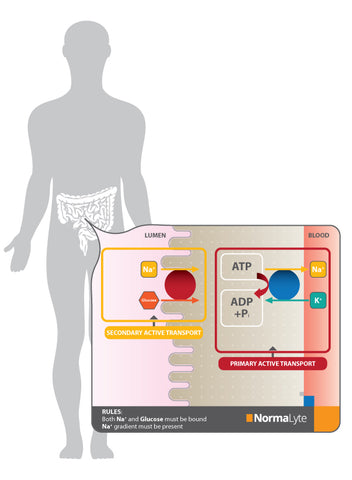
Not as simply put... NormaLyte is uniquely formulated for transformative results through the science of osmosis. Osmosis works inside your intestinal cells to transport glucose, sodium, and water inside each of your cells to later be used as the engine oil to your body. This is called sodium/glucose cotransport, and you can read more about that process in this study.
The formulation of NormaLyte’s solution allows for absorption of water on a 1:1 molar basis in the small intestine. The sodium and potassium are needed to replace the body losses of these essential ions during dehydration. Citrate reduces the acidosis that occurs alongside diarrhea and dehydration. The refreshing flavors in some NormaLyte helps improve the taste, all without preservatives.
With a formulation that targets the most effective molecular processes, NormaLyte’s solution has the right balance of glucose and salts to maintain a 254 mOsm per Liter, which is efficiently absorbed into the body on a molecular level, using osmosis. It contains the amount of glucose and electrolytes recommended by the WHO, and follows the research showing that oral rehydration solutions that contain glucose are more effective than those with low levels of salts.
NormaLyte is more efficient, lower in calories, and better researched in rehydration than sports drinks. With all the added fructose and sucrose, sports drinks can actually be hypertonic, which worsens dehydration with a higher osmolarity than that of the small intestine, pulling water out of the cells.
MAKING WATER BETTER FOR YOU.
While healthcare providers understand that water is essential to health, two thirds of Americans do not drink enough water on a daily basis[1] . Dehydration, especially from illness, is such a concern worldwide that the World Health Organization is targeting diarrhea and dehydration from it as a priority for ending preventable disease deaths by 2025[2].
With its implications on health, having a reliable, expedient dehydration treatment is crucial for healthcare professionals. NormaLyte provides the most reliable treatment available.
THE FOREMOST ORS FORMULATION.
The formulation NormaLyte uses has the low osmolarity most recently recommended by the World Health Organization and UNICEF for optimal rehydration; however, the use of oral rehydration salts has been a proven and recommended treatment for dehydration for more than two decades[3].
The proof of efficacy has been shown throughout the world by researchers and healthcare providers.
HOW FAST DOES NORMALYTE WORK?

Great question! But it's not a simple answer. There are a lot of factors that come into play regarding how quickly water is absorbed into the bloodstream. When you last ate, how your physiology works, and more are all factors that can impact how quickly water is absorbed. On average with NormaLyte, rehydration can be expected within 5 minutes!
DON'T BE AFRAID TO SALT IT UP!
Salt has a bad wrap sometimes, and for good reason. Over consumption isn't always good for you. But did you know that salt is vital to our everyday health? Your body uses salt to balance fluids in the blood and maintain healthy blood pressure, and it is also essential for nerve and muscle function. Not only that, it it allows water to be absorbed more efficiently.
RELIABLE TREATMENT THE WORLD OVER
- Adults and children with dehydration; mild to severe, determined by the number and severity of symptoms
- Infants with mild to moderate dehydration should be treated under medical supervision with ORT in preference to intravenous rehydration
- Breast-fed infants with dehydration should be given ORT in conjunction with continued breastfeeding
- NormaLyte is not indicated for people with heart disease, kidney disease, and fluid/electrolyte/salt restrictions.






