Benefits of Oral Rehydration on Orthostatic Intolerance in Children with Postural Tachycardia Syndrome (Journal of Pediatric Medicine)

It's important to know the facts when you are treating patients for Postural Orthostatic Tachycardia Syndrome. Here's what we know:
The WHO Recommendation
The World Health Organization has outlined the best methods t0 prevent dehydration in individuals with water loss due to chronic illnesses or acute illness like diarrhea. Fairly recently the WHO outlined a new formula that has revolutionized the efficiency of rehydration. Some have hailed it as the most significant discovery in modern medicine.
In the WHO's discovery there is a very specific recipe for oral rehydration. You can read the white papers on ORS (oral rehydration salts) for dehydration here.
NormaLyte follows the WHO recommendations exactly.
NormaLyte is Clinically Studied

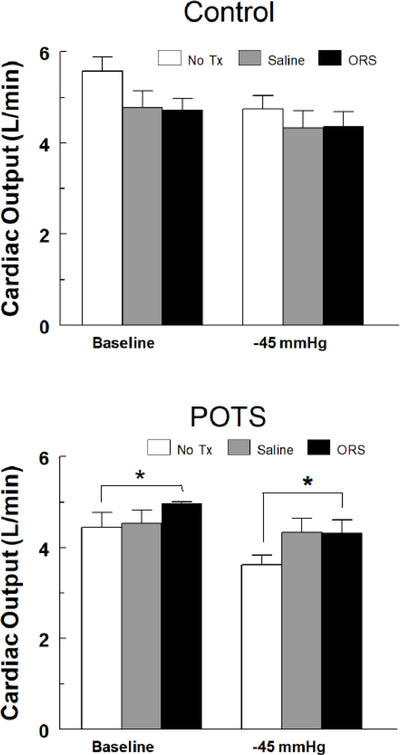
In a clinical study, Oral Rehydration Salts like NormaLyte were proven to be more effective than saline IV alone.
Read the study details to determine if NormaLyte is a good solution for your patients, and view the charts below from the study: